Hanahan lab
We investigate tumor development and progression using genetically engineered mouse models of cancer that recapitulate important characteristics of human cancers, with strategic goals to elucidate pathogenic mechanisms underlying multi-step tumorigenesis and malignant progression, and to develop new therapeutic strategies based on knowledge of mechanism for translation to clinical trials aiming to improve the treatment of human cancers. The lab focuses on melanoma, glioblastoma, pancreatic cancer, and squamous carcinomas. Topics include mechanistic studies on acquired capabilities – hallmarks of cancer – including resistance to programmed cell death, tumor angiogenesis, invasion and metastasis. A crosscutting theme is the role of the heterotypic tumor microenvironment and the accessory cells that collaborate with cancer cells to manifest malignant disease. The lab is also studying mechanisms of adaptive resistance to therapies targeting these and other hallmark capabilities, which present fascinating perturbations into the regulatory systems, and offer potential avenues to circumvent such drug resistance with combinatorial therapies. ...
Research projects
Mechanisms underlying multistep tumor development and their translation toward new therapeutic strategies
The Hanahan lab’s research program is centered upon the use of genetically engineered mouse models (GEMMs) of multistep de novo tumor development, growth, and malignant progression, where we chart pathways of tumorigenesis, seeking to understand the deterministic rate-limiting steps – and their mechanistic underpinnings – that define pathways to cancer. Historically the lab’s research has touched upon most of the hallmarks of cancer, the acquired capabilities thought to be instrumental for the disease. The strategic plan is twofold: 1) to elucidate functional and phenotypic manifestations, molecular and cellular mechanisms, and regulation of acquired hallmark capabilities, and 2) to translate knowledge of mechanisms toward clinical applications, in part via preclinical therapeutic trials that have promise to guide consideration of clinical trials. Currently the lab studies a number of organ-specific cancer models, each with distinctive attributes and potential to impact knowledge and cancer therapy.
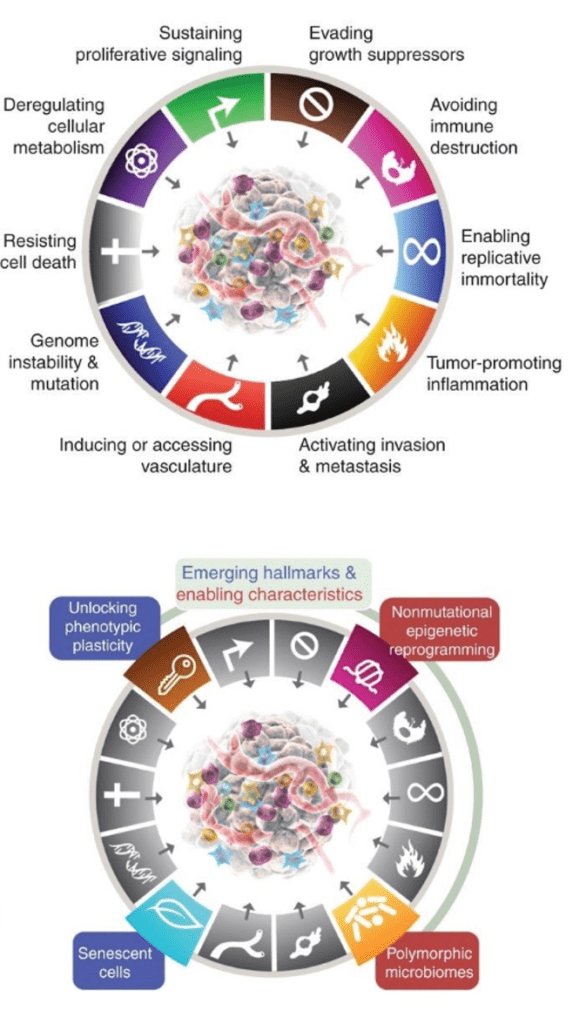
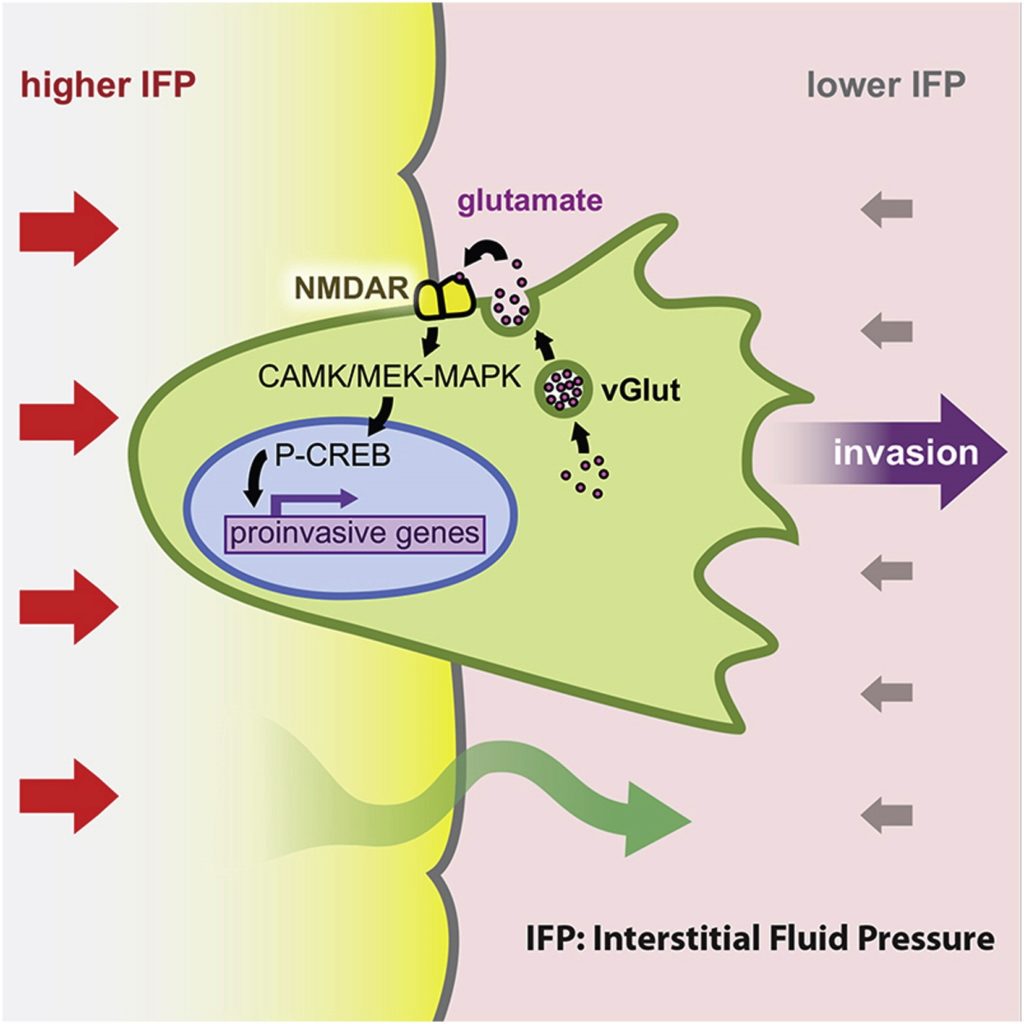
Parameters of the Hallmark Capability for Invasion & Metastasis
The capability for invasion and metastasis acquired during tumor progression stands as the basis for much of the morbidity and mortality associated with cancer. It is also a remarkably diverse and complex parameter. We are studying various facets of this cancer phenotype, in multiple model systems. For example, we discovered a regulatory pathway, which activates invasiveness and is triggered by mechanical forces resultant to differences in hydrostatic pressure between solid tumors and their surrounding tissue, resulting in fluid flow out of the tumor. The pressure-driven fluid flow induces secretion of glutamate, which, in cancer cells expressing the neuronal signaling receptor NMDAR, activates it, stimulating an invasive growth program (Li and Hanahan, 2013). We continue to investigate this invasive growth program in pancreatic and triple negative breast cancer, where it may define a heretofore unappreciated subtype that has coopted glutamate signaling to help drive the malignant phenotype.
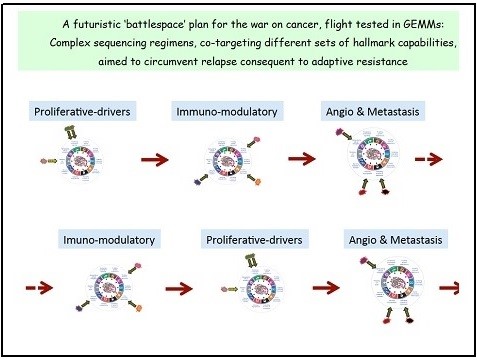
Adaptive Resistance to Mechanism-Targeted Therapies
One fascinating research direction involves elucidating adaptive resistance mechanisms – acquired and intrinsic – to hallmark targeting therapies. The game plan is to identify such mechanisms, and then circumvent them pharmacologically, directly or indirectly. In regard to the latter, we are testing the hypothesis that multi-targeting distinct hallmarks may pose a significant challenge to the evolution of drug resistance, requiring simultaneous adaptations to multiple therapy-impaired hallmarks, thereby improving the duration of treatment response. One can envision, in a future where incipient resistance can be detected early, a war plan involving multi-pronged sequential attacks. The Hanahan lab is flight-testing mechanism-targeted drugs aimed at adaptive resistance as well as hallmark co-targeting strategies, in models of pancreas cancer, melanoma, glioblastoma, and cervical cancer.
Latest publications
Milestones in tumor vascularization and its therapeutic targeting
De Palma M, Hanahan D
Nature Cancer – 2024 Jun 25
Embracing cancer complexity: Hallmarks of systemic disease.
Swanton C, Bernard E, Abbosh C, (...), Wood JN, Vousden KH, Hanahan D
Cell – 2024 Mar 28
Cancer Evolution: A Multifaceted Affair.
Ciriello G, Magnani L, Aitken SJ, (...), Tonon G, Vanharanta S, Zuber J
Cancer discovery – 2023 Dec 6
Cancer hallmarks intersect with neuroscience in the tumor microenvironment.
Hanahan D, Monje M
Cancer cell – 2023 Mar 13
Bispecific PD1-IL2v and anti-PD-L1 break tumor immunity resistance by enhancing stem-like tumor-reactive CD8+ T cells and reprogramming macrophages
Tichet M, Wullschleger S, Chryplewicz A, (...), Umaña P, Klein C, Hanahan D
Immunity – 2023 Jan 10
PD-1-cis IL-2R agonism yields better effectors from stem-like CD8+ T cells.
Codarri Deak L, Nicolini V, Hashimoto M, (...), Ahmed R, Klein C, Umaña P
Nature – 2022 Sep 28
Team

Other members
Selected Publications
Bispecific PD1-IL2v and anti-PD-L1 break tumor immunity resistance by enhancing stem-like tumor-reactive CD8+ T cells and reprogramming macrophages
Tichet M, Wullschleger S, Chryplewicz A, (...), Umaña P, Klein C, Hanahan D
Immunity – 2023 Jan 10
Aberrant hyperexpression of the RNA binding protein FMRP in tumors mediates immune evasion.
Zeng Q, Saghafinia S, Chryplewicz A, (...), Tichet M, Homicsko K, Hanahan D
Science – 2022 Nov 18
Hallmarks of Cancer: New Dimensions.
Hanahan D
Cancer discovery – 2022 Jan
Cancer Cells Retrace a Stepwise Differentiation Program during Malignant Progression.
Saghafinia S, Homicsko K, Di Domenico A, (...), Ciriello G, Michael IP, Hanahan D
Cancer discovery – 2021 Apr 28
A set of microRNAs coordinately controls tumorigenesis, invasion, and metastasis.
Michael IP, Saghafinia S, Hanahan D
Proceedings of the National Academy of Sciences of the United States of America – 2019 Nov 8
A colorectal cancer classification system that associates cellular phenotype and responses to therapy.
Sadanandam A, Lyssiotis CA, Homicsko K, (...), Cantley LC, Gray JW, Hanahan D
Nature medicine – 2013 Apr 14
Hijacking the neuronal NMDAR signaling circuit to promote tumor growth and invasion.
Li L, Hanahan D
Cell – 2013 Mar 28
Accessories to the crime: functions of cells recruited to the tumor microenvironment.
Hanahan D, Coussens LM
Cancer cell – 2012 Mar 20
Hallmarks of cancer: the next generation.
Hanahan D, Weinberg RA
Cell – 2011 Mar 4
Modes of resistance to anti-angiogenic therapy.
Bergers G, Hanahan D
Nature reviews. Cancer – 2008 Aug
Cathepsin cysteine proteases are effectors of invasive growth and angiogenesis during multistage tumorigenesis.
Joyce JA, Baruch A, Chehade K, (...), Hager JH, Bogyo M, Hanahan D
Cancer cell – 2004 May
The hallmarks of cancer.
Hanahan D, Weinberg RA
Cell – 2000 Jan 7
Signaling vascular morphogenesis and maintenance.
Hanahan D
Science (New York, N.Y.) – 1997 Jul 4
Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis.
Hanahan D, Folkman J
Cell – 1996 Aug 9
Differential plasmid rescue from transgenic mouse DNAs into Escherichia coli methylation-restriction mutants.
Grant SG, Jessee J, Bloom FR, Hanahan D
Proceedings of the National Academy of Sciences of the United States of America – 1990 Jun
Transgenic mice as probes into complex systems.
Hanahan D
Science (New York, N.Y.) – 1989 Dec 8
Induction of angiogenesis during the transition from hyperplasia to neoplasia.
Folkman J, Watson K, Ingber D, Hanahan D
Nature – 1989 May 4
Related news
Events
AGORA Sustainability Day
Events
Lola and John Grace Distinguished Lecture in Cancer Research, Prof. Ronald DePinho | June 27th
Events

AGORA PRS | March 26th
Events

PhD Thesis Defense | November 24th
Events
Lola and John Grace Distinguished Lecture in Cancer Research, Prof. Hans Clevers | November 9th
Events

AGORA PRS | May 30th
Events

AGORA PRS | May 16th
Events

AGORA PRS | March 28th
Events

EACR ‘Meet the Author’ webinar with Douglas Hanahan and Agnieszka Chryplewicz | March 9th
Events
Ludwig Distinguished Lecture, Prof. Charles Swanton | January 12th
Events











